|
IEC 80001-1
Edition 2.0 2021-09
INTERNATIONAL
STANDARD
NORME
INTERNATIONALE
colour
inside
Application of risk management for IT-networks incorporating medical devices –
Part 1: Safety, effectiveness and security in the implementation and use of
connected medical devices or connected health software
Application de la gestion des risques aux réseaux des technologies de
l’information contenant des dispositifs médicaux –
Partie 1: Sûreté, efficacité et sécurité dans la mise en œuvre et l'utilisation des
dispositifs médicaux connectés ou des logiciels de santé connectés
your local IEC member National Committee for further information.
Droits de reproduction réservés. Sauf indication contraire, aucune partie de cette publication ne peut être reproduite ni
utilisée sous quelque forme que ce soit et par aucun procédé, électronique ou mécanique, y compris la photocopie et
les microfilms, sans l'accord écrit de l'IEC ou du Comité national de l'IEC du pays du demandeur. Si vous avez des
questions sur le copyright de l'IEC ou si vous désirez obtenir des droits supplémentaires sur cette publication, utilisez
les coordonnées ci-après ou contactez le Comité national de l'IEC de votre pays de résidence.
IEC Central Office Tel.: +41 22 919 02 11
3, rue de
CH-1211 Geneva 20
Switzerland
About the IEC
The International Electrotechnical Commission (IEC) is the leading global organization that prepares and publishes
International Standards for all electrical, electronic and related technologies.
About IEC publications
The technical content of IEC publications is kept under constant review by the IEC. Please make sure that you have the
latest edition, a corrigendum or an amendment might have been published.
IEC publications search - webstore.iec.ch/advsearchform IEC online collection - oc.iec.ch
The advanced search enables to find IEC publications by a Discover our powerful search engine and read freely all the
variety of criteria (reference number, text, technical publications previews. With a subscription you will always have
committee, …). It also gives information on projects, replaced access to up to date content tailored to your needs.
and withdrawn publications.
Electropedia - www.electropedia.org
IEC Just Published - webstore.iec.ch/justpublished
The world's leading online dictionary on electrotechnology,
Stay up to date on all new IEC publications. Just Published
containing more than 22 000 terminological entries in English
details all new publications released. Available online and once
and French, with equivalent terms in 18 additional languages.
a month by email.
Also known as the International Electrotechnical Vocabulary
(IEV) online.
IEC Customer Service Centre - webstore.iec.ch/csc
If you wish to give us your feedback on this publication or need
further assistance, please contact the Customer Service
.
A propos de l'IEC
La Commission Electrotechnique Internationale (IEC) est la première organisation mondiale qui élabore et publie des
Normes internationales pour tout ce qui a trait à l'électricité, à l'électronique et aux technologies apparentées.
A propos des publications IEC
Le contenu technique des publications IEC est constamment revu. Veuillez vous assurer que vous possédez l’édition la
plus récente, un corrigendum ou amendement peut avoir été publié.
Recherche de publications IEC - Découvrez notre puissant moteur de recherche et consultez
webstore.iec.ch/advsearchform gratuitement tous les aperçus des publications. Avec un
La recherche avancée permet de trouver des publications IEC abonnement, vous aurez toujours accès à un contenu à jour
en utilisant différents critères (numéro de référence, texte, adapté à vos besoins.
comité d’études, …). Elle donne aussi des informations sur les
projets et les publications remplacées ou retirées. Electropedia - www.electropedia.org
Le premier dictionnaire d'électrotechnologie en ligne au monde,
IEC Just Published - webstore.iec.ch/justpublished
avec plus de 22 000 articles terminologiques en anglais et en
Restez informé sur les nouvelles publications IEC. Just
français, ainsi que les termes équivalents dans 16 langues
Published détaille les nouvelles publications parues.
additionnelles. Egalement appelé Vocabulaire
Disponible en ligne et une fois par mois par email.
Electrotechnique International (IEV) en ligne.
Service Clients - webstore.iec.ch/csc
Si vous désirez nous donner des commentaires sur cette
publication ou si vous avez des questions contactez-nous:
[email protected] .
IEC online collection - oc.iec.ch
IEC 80001-1
Edition 2.0 2021-09
INTERNATIONAL
STANDARD
NORME
INTERNATIONALE
colour
inside
Application of risk management for IT-networks incorporating medical devices –
Part 1: Safety, effectiveness and security in the implementation and use of
connected medical devices or connected health software
Application de la gestion des risques aux réseaux des technologies de
l’information contenant des dispositifs médicaux –
Partie 1: Sûreté, efficacité et sécurité dans la mise en œuvre et l'utilisation des
dispositifs médicaux connectés ou des logiciels de santé connectés
INTERNATIONAL
ELECTROTECHNICAL
COMMISSION
COMMISSION
ELECTROTECHNIQUE
INTERNATIONALE
ICS 11.040.01; 35.240.80 ISBN 978-2-8322-9748-3
– 2 – IEC 80001-1:2021 © IEC 2021
CONTENTS
FOREWORD . 4
INTRODUCTION . 7
1 Scope . 9
2 Normative references . 9
3 Terms and definitions . 9
4 Principles . 10
5 Framework . 11
5.1 General . 11
5.2 Leadership and commitment . 11
5.3 Integrating RISK MANAGEMENT . 11
5.4 Design/planning . 12
5.4.1 General . 12
5.4.2 RISK MANAGEMENT FILE . 13
5.4.3 Understanding the organization and the SOCIOTECHNICAL ECOSYSTEM . 13
5.4.4 Articulating RISK MANAGEMENT commitment . 13
5.4.5 Assigning organizational roles, authorities, responsibilities and
accountabilities . 13
5.4.6 Allocating resources . 14
5.4.7 Establishing communication and consultation . 14
5.5 Implementation . 15
5.6 Evaluation . 15
5.7 Improvement . 15
6 RISK MANAGEMENT PROCESS . 15
6.1 Generic requirements. 15
6.1.1 General . 15
6.1.2 RISK ANALYSIS . 16
6.1.3 RISK EVALUATION . 18
6.1.4 RISK CONTROL . 19
6.2 Lifecycle specific requirements . 21
6.2.1 General . 21
6.2.2 Acquisition . 21
6.2.3 Installation, customization and configuration . 22
6.2.4 Integration, data migration, transition and validation . 22
6.2.5 Implementation, workflow optimization and training . 22
6.2.6 Operation and maintenance . 23
6.2.7 Decommission . 24
Annex A (informative) IEC 80001-1 requirements mapping table . 25
Annex B (informative) Guidance for accompanying document Information . 31
B.1 Foreword . 31
B.2 Information system categorization . 32
B.3 Overview. 32
B.4 Reference documents . 32
B.5 System level description . 32
B.5.1 Environment description . 32
B.5.2 Network ports, protocols and services . 33
B.5.3 Purpose of connection to the health IT infrastructure . 33
B.5.4 Networking requirements . 33
B.5.5 Required IT-network services . 33
B.5.6 Data flows and protocols . 33
B.6 Security and user access . 34
B.6.1 General . 34
B.6.2 Malware / antivirus / allow-list . 34
B.6.3 Security exclusions . 34
B.6.4 System access . 34
B.7 RISK MANAGEMENT . 36
Bibliography . 37
Figure 1 – Lifecycle framework addressing safety, effectiveness and security of health
software and health IT systems . 8
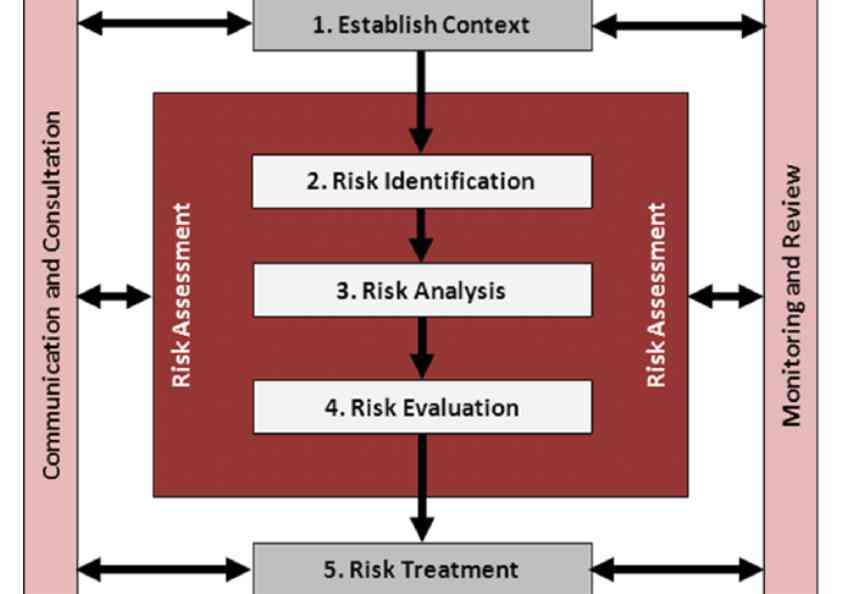
Figure 2 – RISK MANAGEMENT PROCESS . 12
Table A.1 – IEC 80001-1 requirements table . 25
Table B.1 – Organization name and location . 31
Table B.2 – Cybersecurity device characterization level . 32
Table B.3 – Ports, protocols and services . 33
Table B.4 – Information system name and title . 34
Table B.5 – Roles and privileges . 35
– 4 – IEC 80001-1:2021 © IEC 2021
INTERNATIONAL ELECTROTECHNICAL COMMISSION
____________
APPLICATION OF RISK MANAGEMENT FOR IT-NETWORKS
INCORPORATING MEDICAL DEVICES –
Part 1: Safety, effectiveness and security in the implementation and use
of connected medical devices or connected health software
FOREWORD
1) The International Electrotechnical Commission (IEC) is a worldwide organization for standardization comprising
all national electrotechnical committees (IEC National Committees). The object of IEC is to promote international
co-operation on all questions co
...